This is the fourth in an in-depth multi-part blog series on how and why this amino acid is so frequently prescribed and used off-label as an ADHD treatment method. Reviews and literature findings are mixed, but some physicians (and parents and individuals with ADHD themselves) swear by tyrosine as a hugely successful treatment strategy for ADHD. We have spent the last three posts examining:
- The different enzymes and enzyme systems used in tyrosine metabolism
- Which (if any) nutrient "helpers" or "co-factors" are required by these enzyme systems to function properly, and
- The implications these have on the neuro-biology of ADHD
 As a quick recap:
As a quick recap:- In tyrosine and ADHD post #1, we gave a general overview of the process and the roles of dopamine and norepinephrine on ADHD biology. We also looked at how tyrosine enters the brain, and which mechanisms are important for facilitating its transport to the desired targets for therapeutic effects with regards to ADHD (Please note that different forms of tyrosine exist, but the form most common in nature and in chemistry in general is referred to as "L-tyrosine". When this blog mentions "tyrosine", it is this "L" form we are referring to in all cases unless specified otherwise).
- In the second post on ADHD and tyrosine, we focused on the first step of the process, the conversion of tyrosine to L-DOPA. This step heavily utilizes a specific enzyme called tyrosine hydroxylase. Tyrosine Hydroxylase is dependent on adequate supplies of certain nutrients such as iron, magnesium, zinc, tetrahydrobiopterin, and adequate levels of vitamin C (and antioxidants in general). While rampant supplementation is not necessary, inadequate levels of any of these agents (as well as a few others, such as copper) could potentially compromise the function of the tyrosine hydroxylase enzyme. It is important to note that the conversion of tyrosine to L-DOPA is typically the slowest and rate-limiting step of the whole tyrosine metabolism and conversion process to dopamine and norepinephrine. Thus, compromising this first conversion step can be potentially the most devastating with regards to impaired tyrosine metabolism for ADHD. This was why the post was a bit lengthy with regards to advocating for nutritional sufficiency.
- The third post on tyrosine and ADHD focused more on the question as to whether we could bypass the first step of the chemical process outlined above entirely by supplementing with L-DOPA (the second major step of the tyrosine conversion process) directly. We discussed the pro's and con's of using each (tyrosine or L-DOPA) as a starting point for ADHD treatment.
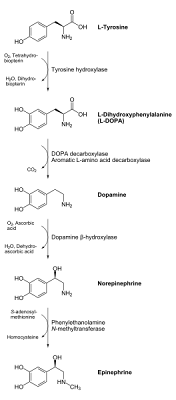
DOPA decarboxylase belongs to a particular class of enzymes called aromatic amino acid decarboxylases. The term" aromatic" here refers to a particular type of "ring" structure in the chemical compound (if you don't have a background in organic chemistry, take a look at the chemical depictions of tyrosine, L-DOPA and dopamine shown below:

***A quick note on the chemical processes shown above and below: If you're not a chemist, don't worry, just look at what's changing in the pictures above and below, which represents the chemical structure of these different molecules involved in the tyrosine to dopamine conversion process. That hexagon-like structure on the left side of these molecules, (with the -OH groups coming off of it) is what makes these compounds "aromatic".
The enzyme tyrosine hydroxylase simply adds another "-OH group" to the top-left side this hexagonal ring to make L-DOPA out of tyrosine. The chemical process of this conversion was the point of discussion in our second blog post on ADHD and tyrosine supplementation. Our next enzyme-driven step leaves this "aromatic" hexagonal ring alone, and instead works on chemically modifying the right side of the molecule, as we'll see in a second. ***
The term originally comes from the fact that chemicals with this type of built-in structure often gave off a particular aroma. Aromatic amino acid decarboxylases essentially take a carbon dioxide off of these six-membered rings, which greatly changes the chemical properties and reactivity of the chemical compound in most cases. (Do you see how the right end of the molecule L-DOPA is "chopped off" to get to dopamine in the step shown below? That is the work of these decarboxylase enzymes).

Of these decarboxylase enzymes (there are several different variations), the "best" one for this conversion process is called DOPA decarboxylase.
Although DOPA decarboxylase can be indirectly affected by several different nutrients (specifically shortages of nutrients), the main one involved in this step is called pyridoxal phosphate. Pyridoxal phosphate is the chemically "active" form of vitamin B6.
We have spoken about the merits of vitamin B6 with regards to ADHD and how it works in conjunction with other nutrients in previous posts. For example, getting B6 into this desired pyridoxal phosphate form requires zinc (another reason why adequate zinc levels are necessary for optimal tyrosine metabolism). It also appears that vitamin B6 works well alongside magnesium as an ADHD treatment combination strategy. Finally, vitamin B6 plays a role in the metabolism of omega-3 fatty acids (omega-3 rich fish oil is a common "natural" treatment method for ADHD)
Because of its vital role as a "co-factor" or "helper" of the DOPA decarboxylase enzyme, which is responsible for converting L-DOPA to dopamine, it is imperative that we avoid shortages of this essential B vitamin. A rough estimate of recommended daily intake levels of vitamin B6 can be found here. Keep in mind that over 100 different other enzymes also depend on vitamin B6 and its derivatives, so keeping adequate stores of this vitamin is essential.
In addition to keeping up necessary vitamin B6 levels to help the DOPA decarboxylase enzyme's ability to function properly in the second major chemical step of tyrosine metabolism, we must also mention an often-overlooked issue with the enzyme: the interaction of DOPA decarboxylase with another common neurochemical signaling agent called serotonin.
Serotonin is generated from another important amino acid called tryptophan. Tryptophan (like tyrosine) is an aromatic amino acid, and the two amino acids have several structural and functional similarities. While this may sound like a good thing at first, it can lead to some problems.
One of these problems is the fact that if two chemicals share similar structural characteristics, enzymes which act on one may also act on the other. If the structural characteristics are close enough, the two agents can even compete for the same enzymes, or effectively block each other off or crowd each other out.
This is precisely what can happen with the amino acid tryptophan and its product serotonin. The tryptophan to serotonin process also uses these aromatic amino acid decarboxylase enzymes (and interestingly, also uses vitamin B6 as a cofactor in the process. This is yet another reason why we want to keep B6 levels up to speed!).
**A generalized conversion process of tryptophan to serotonin is shown below. Note that this pathway is analogous to the tyrosine to dopamine pathway in a number of ways, including the addition of a hydroxyl (-OH) group in the first step and a decarboxylation (essentially the removal of carbon dioxide) in the second step, which utilizes both the aromatic amino acid decarboxylase enzymes and pyridoxal phosphate (vitamin B6). Do you see how these two processes can easily be in competition with each other for resources (the enzymes as well as the vitamin B6).
 Additionally, the end product of the above process, serotonin, can also effectively shut the enzyme DOPA decarboxylase down. This process, in which an enzyme is essentially shut down by its final products, is often used in the body to keep from overproducing one particular kind of substance. It is known as feedback inhibition, and is a very common and crucial process for retaining chemical balances in the body.
Additionally, the end product of the above process, serotonin, can also effectively shut the enzyme DOPA decarboxylase down. This process, in which an enzyme is essentially shut down by its final products, is often used in the body to keep from overproducing one particular kind of substance. It is known as feedback inhibition, and is a very common and crucial process for retaining chemical balances in the body.However, if large amounts of tryptophan are present, not only can the crowd out tyrosine for the dopa decarboxylase enzyme, but the final product of this tryptophan (serotonin), can essentially shut the enzyme down for both processes. In other words, it's a double-whammy for tyrosine, along with the implications for its use as an ADHD treatment strategy.
Actually, make that a triple-whammy. Remember how we mentioned that chemical compounds of similar structure can often crowd each other out? It turns out that tyrosine and tryptophan both compete with each other for transport into the brain. In the first post on this topic, we talked about the blood brain barrier, and how crossing this biochemical barrier was needed to successfully deliver the drug or nutrient-based treatment to the desired brain regions.
This is not meant to blast tryptophan or serotonin. Both chemicals are crucial to a number of important bodily functions. Rather, it is the timing of the administration of these nutrients with which we should be careful. The main strategy here is to try to avoid taking tryptophan-rich foods alongside tyrosine supplements. Some foods which are high in tryptophan can be found here. Keep in mind, however, that many of these tryptophan-rich foods may also be high in tyrosine (such as wild game and several types of seeds like pumpkin seeds). Some of the more tryptophan-concentrated foods are milk, turkey, and legumes (chick peas, peanuts, etc.), so it would be a good idea to refrain from these rich sources of tryptophan for a couple of hours on either side of tyrosine supplementation.
So with regards to the second major step of tyrosine supplementation, the conversion of L-DOPA, we should remember these 2 main things:
- Keep up adequate levels of vitamin B6 to help the DOPA decarboxylase enzyme function at peak efficiency.
- Try to avoid taking in tryptophan-rich foods anytime near the time you take your tyrosine supplements. This will help you avert most of the competitive biochemical processes between these two nutrients, and can ultimately improve the efficacy of tyrosine as an ADHD treatment strategy.



0 comments:
Post a Comment