In previous blog posts, I have mentioned some unconventional and lesser-known
medications used to treat ADHD. Many are either new to the market or have primary uses not designated as ADHD drugs, such as anti-depressants, mood-stabilizers, anti-convulsants, etc. Unfortunately, these results are often obscured or hidden from the general public. The medical community (somewhat understandably) often initially shies away from these studies because they are often done on a small scale, have less-rigorous built-in-controls, are not done by big-name researchers, are studied in foreign countries, and are published in less-prominent journals. What is often surprising is that the results of treatment with these less-publicized medication choices, is that, although small and somewhat isolated in nature, a number these studies have displayed eye-opening levels of success, and should warrant further investigation.
The beauty of being a blog-writer, as opposed to a highly-publicized journalist, is that one can take more of a "chance" by reporting some of these findings, without feeling pressured to stick to the more "mainstream" findings.
Without further ado, the drug of topic for today is
Reboxetine.
Like many ADHD drugs, Reboxetine (also marketed under labels such as
Solvex,
Prolex,
Vestra,
Davedax,
Edronax or
Norebox). It's main line of treatment is for depressive and panic disorders, but has also shown solvency in the treatment of ADHD on a small-scale. Like many other ADHD medications, Reboxetine exists as a mixture of two compounds, which are mirror-images (also called
enantiomers), of each other. It is used in a number of European countries, but is yet to be approved in the United States.
Functionally, and to a lesser-degree, chemically, Reboxetine resembles another common ADHD medication,
Strattera (
Atomoxetine). Unlike many types of anti-depressant medications, which often target the key neuro-signaling agent serotonin, Reboxetine's primary target is another major signaling compound known as
norepinephrine. Norepinephrine, a chemical "cousin" to adrenaline, is often found to be at lower-than-normal levels in the surrounding environment outside neuronal cells in individuals with attentional and depressive (in addition to other related) disorders. Essentially, there is an imbalance in the amount norepinephrine inside and outside the cells on the nervous system. Reboxetine functions as a "blocker" of the process of taking norepinephrine up into neuron cells, which helps restore the balance of this neurotransmitting agent inside and outside cells in the nervous system.
This selective restoration of balance concerning levels of norepinephrine serves other benefits as well. For example, disorders such as
fibromyalgia and
chronic pain are associated with norepinephrine level imbalances. Based on multiple case studies, it appears that
reboxetine can help alleviate at least some of these pain-related symptoms. Attentional deficits are often (perhaps, not surprisingly) a secondary symptom of pain-related disorders, so this is of some therapeutic value already. Additionally, migraine headache pain is also a common comorbid symptom of ADHD. However, there is more...
One of the most difficult issues surrounding drug design is specificity. We naturally want the drug to reach its desired target in the body. However, it is often difficult for a drug to reach only its specific target and avoid all other undesired ones. Unfortunately, this is not always possible, and one of the main consequences of a drug's lack of selectivity is unwanted side effects. In the case of Reboxetine, however, it appears that its overall degree of affinity for unwanted targets (often referred to as
receptors in biological terms) is less than many other comparable medications. In other words, Reboxetine is less "promiscuous"; it has minimal interaction with target receptors for other neurotrasmitters such as
acetylcholine (which can lead to digestive dysfunction, and is partly responsible for the dry-mouth and constipation symptoms found in many drugs) and
serotonin (which can lead to drowsiness and other sedative effects).
Returning to the specific topic of ADHD, however, Reboxetine has shown to have some other advantages over other ADHD medications.
- Reboxetine is long-lasting. Reboxetine's plasma half-life is around 13 hours (that is, it takes around 13 hours for half of the drug to be cleared and eliminated in the body). In comparison, atomoxetine (Strattera) has a plasma half-life of around 4 hours.
- While some medications have shown to be effective in treating the predominantly inattentive symptoms of ADHD or the hyperactive-impulsive symptoms of the disorder, Reboxetine appears to improve symptoms of both. Based on a study of boys ages 6-16 of the Combined subtype (that is, they show significant levels of inattentive as well as hyperactive and impulsive symptoms), treatment with Reboxetine showed significant improvements based on parent ratings in as little as 2 weeks.
- While specificity in choice of biological targets appears to be an advantage of Reboxetine, it also appears that Reboxetine can also boost free dopamine levels in the prefrontal cortex region of the brain (which is a region thought to be highly-connected to ADHD). Dopamine is another highly important agent used in signaling throughout the nervous system and its cells, and is intricately connected with ADHD in the prefrontal cortex region of the brain (which is located behind the forehead). Reduced levels of dopamine in between nerve cells in this important region of the brain (like the lower levels of norepinephrine described above), typically results in an increased onset of negative ADHD symptoms. These effects are thought to be more indirect, as norepinephrine carriers can also transport and clear dopamine from the areas in between neuron cells. However, if these carriers are tied down or "busy" handling the Reboxetine, then these carriers are less available to shuttle away the free levels of dopamine in this critical brain region. As a result, a gradual build-up to more "normal" levels of dopamine are seen, which often results in a reduction of ADHD symptoms.
Other interesting points of note regarding Reboxetine:
- As mentioned above, Reboxetine was rejected by the FDA in the United States, although it has been used widely in over 50 other countries. The reasons for its rejection by the FDA have not been disclosed in full to the general public.
- While the study mentioned above cited the effectiveness of Reboxetine treatment for some children who had experienced adverse side effects with methylphenidate, around half of the children in the study who showed negative side effects to methylphenidate also saw similar effects to Reboxetine (although many were more mild than for methylphenidate).
- While Reboxetine does not target serotonin receptors like many other antidepressant medications (which can cause sedative effects), drowsiness is still one of the more common side effects of the drug. Additionally, treatment with Reboxetine can also lead to appetite suppression, which is a common side effect of stimulant medications used to treat ADHD.
- While dopamine is the main agent of concern in the prefrontal cortex region of the brain with regards to the disorder ADHD, norepinephrine levels in this brain region are thought to be connected to oppositional behavior. While this study used atomoxetine for treating these symptoms (albeit in a rat model), it leaves the door open for investigation of treatment with atomoxetine or reboxetine for both ADHD along with comorbid conduct disorders such as Oppositional Defiant Disorder (ODD, which is actually quite common in ADHD individuals).
- Reboxetine is metabolized mainly in the liver, using an enzyme called CYP3A4. Several other drugs and food compounds also utilize this enzyme system. This is important because when two or more drugs or food-substances share a similar pathway, there is a much greater potential for these substances to interfere with each other. The result is often impairments or drug-drug interactions. For a comprehensive list of other types of drugs and compounds which also use this enzyme system, please click here. Although not emphasized in the previous link, I personally found it interesting that the compound quercetin was a strong inhibitor of this enzyme system. Quercetin is found in high concentrations in foods such as onions, teas, apples, and berries, many of which are touted for their numerous health benefits such as cardiovascular health and antioxidant properties. While no significant studies (at least to the best of this writer's knowledge), have been done on the effects of quercetin and the drug Reboxetine, there is a strong possibility that high levels of consumption of these healthy antioxidant-rich foods may actually interfere with the metabolism of Reboxetine and potentially alter its effectiveness in treating ADHD or related disorders.
In spite of a number of positive findings surrounding the drug, there is still a shroud of mystery (much of which is due to the FDA rejection of the drug in the U.S.) over the effectiveness of Reboxetine for treating ADHD on a large scale. Given the fact that its main function is that of an antidepressant, it would appear that functionally, Reboxetine would be useful for treating individuals with ADHD and comorbid depression (in a way somewhat analogous to drugs such as Wellbutrin).
Nevertheless, some of the promising results surrounding the drug suggest a potential for treatment of comorbid conduct disorders. This may serve as a potential all-in-one approach, as opposed to being prescribed multiple drugs for multiple co-existing symptoms. The versatility of this drug is intriguing, especially when we consider the relative specificity that Reboxetine has almost exclusively for the signaling agent norepinephrine.
Given the fact that this class of antidepressants appears to bypass the serotonin-dependent pathways, it is possible that this drug could be used in conjunction with other anti-depressant drugs as well, with a reduced potential for negative drug-drug interference.
Finally, due to its comparatively long half-life, and potential interference from foodstuffs such as quercetin, there is an increased risk of unwanted buildup and possible side effects associated with toxicity issues surrounding the drug. Nevertheless, there is room for further exploration, especially in the context of approaching ADHD treatment from a different angle than most stimulant medications. This is definitely a drug to keep on the radar for the near future.






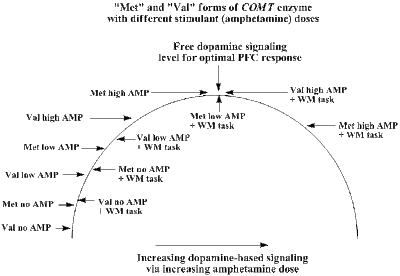 From here we should be able to spot three trends:
From here we should be able to spot three trends: